Protocol for Western & IP of mCFTR 3G11 rat monoclonal against mouse CFTR
The Scripps Research Institute
Department of Cell Biology, MB6
10550 N. Torrey
Tissue Culture Cells: Preparation of Lysate
CHO (Riordan) expressing mouse wild type CFTR cells were harvested as follows:
Cell are prepared ahead of time as follows:
1. Wash 150mm dishes with cold PBS.
2. Scrape in cold PBS.
3. Aliquot to 1.5ml the equivalent of a 60mm dish
(ie 7.2samples/150mm dish, 3 samples/100mm dish, 1sample 60mm dish)
3. Spin briefly in refrigerated microfuge to pellet cells.
4. Aspirate supernatant.
5. Freeze tubes at –80°C.
Western Blot
1. Block with TBS/0.1%Tween/4%Milk
2. Add 3G11 (rat monoclonal) at 2.5ug/ml in TBS /0.1%Tween /4% milk 1hr RT or 4°C overnight.
3. Wash with TBS/Tween 3x 30 min.
4. Goat anti-Rat HRP at 1:10, 000 in TBS/Tween 1hr RT.
(
5. Wash TBS/Tween 3x 30min.
6. Develop with luminal/H202 reagents.
Use ~30-50 mg of CHO lysate per lane for detection (30-60 sec exposure).
Immunoprecipitation Protocol
Tubes thawed on ice with the addition of 270 ml of lysis buffer/6.0 mm dish (protein concentration is about 1.5-3mg/ml).
Lysis buffers:
(A) Triton lysis buffer (TLB) (1% Triton, 25mM Tris pH 7.4, 150mM NaCl + protease tablets at 2 mg/ml).
(B) RIPA lysis buffer (RLB) (1% Triton, 150mM NaCl, 25mM Tris pH 7.4, .1% SDS, 0.5% deoxycholate + protease tablets (Roche) at 2mg/ml).
- After addition of lysis buffer, leave on ice for about 10-15 min.
- Spin 14,000 rpm (20,000g) for 20 min.
- To supernatant add 30 ml of 50% washed protein G beads to pre-clear the lysate.
- Incubate ~60 min at 4°C with rocking.
- Spin out beads (20 s top speed in microfuge).
- Spin at 100,000 x g in the ultracentrifuge at 4°C for 20 min to remove insoluble aggregates.
- Transfer supernatant to a 1.5 ml tube containing 25-35ul of a 50% suspension of 3G11 beads covalently coupled at 4mg/ml to protein G beads. (Alternatively- add Ab separately for 45 min then add protein G beads overnight - we have not tested this condition and would need to determine how much antibody to add). We usually start testing at 10ug/ml.)
- Incubate overnight with rocking at 4°C.
- Wash 2X with TLB and 1X with TLB minus detergent.
- Add 14 ml of 2X gel sample buffer (Laemmli) containing 50 mM DTT.
- Heat at 37°C for 20 min. Note that CFTR precipitates and becomes insoluble if the sample is heated above 60°C.
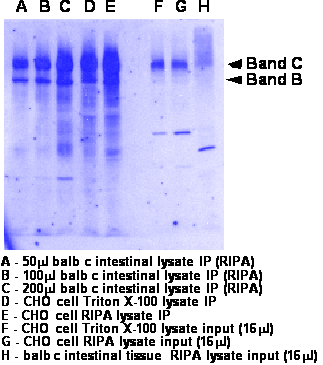
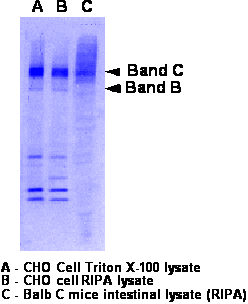
- Run 8% gel and transfer to nitrocellulose. Should migrate as a doublet at 140kDa (band B- weak band) and 160kDa (band C-strong band)
- Western was performed as described above. Either 3G11 at 2ug/ml or M3A7 at 1:2500 was used in TBS/Tween/milk. Goat anti rat or goat anti mouse are 1:10,000 in TBS/Tween. See blot for more info.
- Western is performed as described above.
- Blot is probed with rabbit polyclonal at 1:2500-3000 since rat 3G11 was used for IP.
Mouse Intestinal
Epithelial Cells
Cells are prepared following the procedure from Lawson and Powell, Am J. Physiol: G783-G790, 1987 as described to us by Lane Clark.
- Thaw intestinal epithelial cells on ice. (Cells resuspended in PBS before freezing). Note: as judged from Western blot, this stage is highly susceptible to proteolysis of CFTR. Important to rapidly freeze in liquid nitrogen upon dissection, followed by rapid thaw and lysis. We currently trying to optimize thawing conditions by solubilizing directly in lysis buffer to get protease inhibitors quickly targeted to lysed cells).
- Spin 2500 rpm for 10 min at 4°C in a table-top centrifuge.
- Discard supernatant (it will be cloudy) and add three pellet volumes of RLB buffer to the pellet (see above) and vortex vigorously. Note: can also use TLB with lower yields on mouse tissue. TLB readily applicable to CHO cells expressing mouse CFTR)
- Lyse on ice for 15 min with intermittent vortexing.
- After 15 min, use a 23G needle with syringe and go up and down about 5X to further disrupt clumps.
- Proceed as above from step 2 for immunoprecipitation.